Global Life Sciences
Overview
Featured News & Insights
0016
Contacts
Services
Advising on the full scope of regulatory, compliance and enforcement, litigation and transactional matters, we can offer you a holistic legal approach that will smooth your path as you navigate industry changes, technological advances, market demands and policy hurdles.
Regions
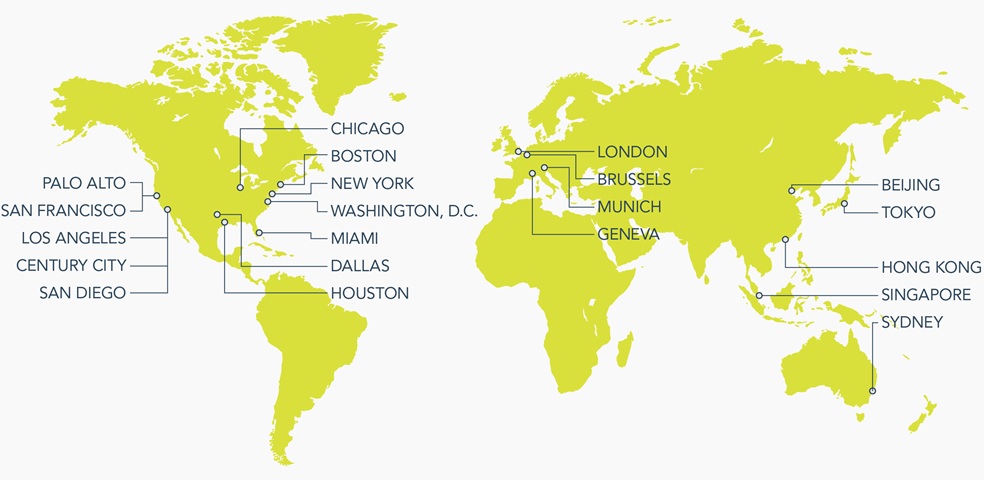
From high-profile transactions to regulatory issues and complex litigation, we collaborate across borders and disciplines to help our clients in more than 70 countries face challenges and embrace opportunities.
All Professionals
sort


Capabilities
- Antitrust and Competition
- Capital Markets
- Commercial Litigation and Disputes
- Emerging Companies and Venture Capital
- Environmental, Health, and Safety
- Food, Drug and Medical Device
- Global Arbitration, Trade and Advocacy
- Government Strategies
- Healthcare
- IP Litigation
- M&A
- Privacy and Cybersecurity
- Private Equity
- Product Liability and Mass Torts
- Technology
- Technology and Life Sciences Transactions
Related Resources
Related Blogs
News & Insights
Sidley Sponsors the 2026 EU Pharma Law ForumTuesday, May 19, 2026 – Thursday, May 21, 2026Women's Life Sciences Network: EU Pharmaceutical Law ForumTuesday, May 19, 2026Inside Strategic Partnerships: Clinical Trial Considerations and AI/Digital Health InsightsThursday, March 12, 2026New U.S. DOJ Antitrust Leadership Signals More Criminal Prosecutions and Longer Prison SentencesMarch 6, 2026
Events
Events
Conferences
Antitrust and White Collar Defense and Investigations Update
New U.S. DOJ Antitrust Leadership Signals More Criminal Prosecutions and Longer Prison SentencesMarch 6, 2026Generative AI and Privilege: Practical Lessons from Two Early Decisions and What Comes NextFebruary 27, 2026New York LLC Transparency Act Took Effect January 1, 2026; U.S.-Formed LLCs Exempt from Reporting ObligationsFebruary 25, 2026Direct-to-Consumer Prescription Drug Platforms: New Government Guidance and Comment OpportunityFebruary 24, 2026
Antitrust and White Collar Defense and Investigations Update
Artificial Intelligence Update
M&A Update
Global Life Sciences Update
Drug Pricing and Medicaid Rebate Reporting: Enforcement Risk RemainsFebruary 17, 2026Legal Issues Arising From the Use of Artificial Intelligence in Drug DevelopmentFebruary 11, 2026DOJ Announces Renewal of FCA Working GroupOctober 2025False Claims Act Liability: When Do Claims for Health Care Reimbursement 'Result From' Anti-Kickback Statute Violations?September 25, 2025
Reuters
Drug Discovery World
Pratt’s Government Contracting Law Report
Reuters
Sidley Sponsors the 2026 EU Pharma Law ForumTuesday, May 19, 2026 – Thursday, May 21, 2026Women's Life Sciences Network: EU Pharmaceutical Law ForumTuesday, May 19, 2026Inside Strategic Partnerships: Clinical Trial Considerations and AI/Digital Health InsightsThursday, March 12, 2026Life Sciences College 2026Thursday, February 12, 2026
Events
Events
Conferences
Conferences
Sidley Represents Precision NeuroMed in Its Strategic Partnership With Brainlab SEFebruary 27, 2026Sidley Represents Sensei Biotherapeutics in Acquisition of Faeth TherapeuticsFebruary 18, 2026Law360 Profiles Sidley as a 2025 Compliance Group of the YearFebruary 18, 2026Sidley Represents Arsenal Capital Partners and MaxHealth in Sale of MaxHealth to CenterWellFebruary 17, 2026
Announcements
Announcements
Announcements
Announcements
Sidley Recognized by China Business Law Journal for Eight 2025 “Deals of the Year”March 5, 2026Sidley’s Private Equity Capabilities Recognized in Chambers Germany 2026February 19, 2026Sidley Shortlisted for Deal of the Year and Attorney of the Year at D CEO Mergers & Acquisition Awards 2026February 17, 2026Sidley Commended in Chambers Global 2026February 12, 2026
Accolades
Accolades
Accolades
Accolades
TX AG’s Office Continues Aggressive Enforcement Against Healthcare Entities Operating in TexasFebruary 25, 2026DAAG Brenna Jenny Warns Heightened FCA Enforcement Is “The New Normal,” Addresses Enforcement Priorities and PoliciesFebruary 20, 2026Delaware Supreme Court Makes Earnouts Less Risky For Buyers Earnout Decision Partially Reversed Because Buyer Did Not Have to Pursue an Alternative Regulatory PathwayJanuary 27, 2026FY 2025 FCA Settlements and Judgments Statistics Show Highest Recoveries EverJanuary 20, 2026
Original Source
Original Source
GoodLifeSci
Original Source
Renewed Market Optimism @ JPM 2026January 12, 2026What's Ahead for Life Sciences Dealmaking @ JPM 2026January 12, 2026Top 10 FDA Areas We Are Watching @ JPM 2026January 12, 2026Regulatory Considerations for the Next Wave of Development-Stage Transactions @ JPM 2026January 12, 2026
Videos
Videos
Videos
Videos